Information on current therapies
An overview of the current therapy concepts with gene therapy, haematopoietic stem cell transplantation (bone marrow transplantation), enzyme replacement therapy, substrate reduction therapy and chaperone therapy is scientifically presented in the following article:
Pinto, E. Vairo F., D. Rojas Malaga, F. Kubaski, C. Fischinger Moura de Souza, F. de Oliveira Poswar, G. Baldo, and R. Giugliani. "Precision Medicine for Lysosomal Disorders" Biomolecules 10, no. 8 https://dx.doi.org/10.3390/biom10081110 (Jul 26 2020).
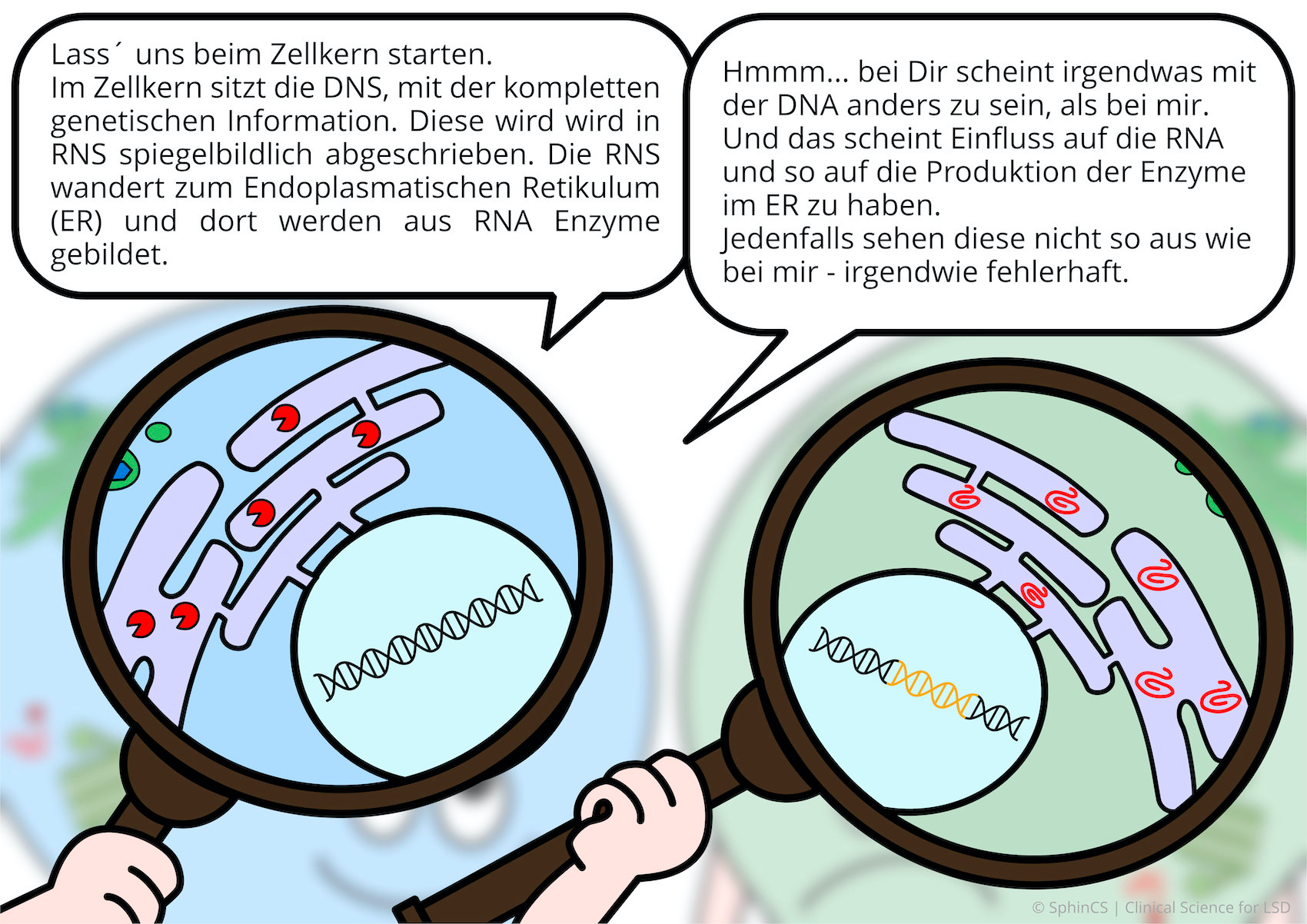
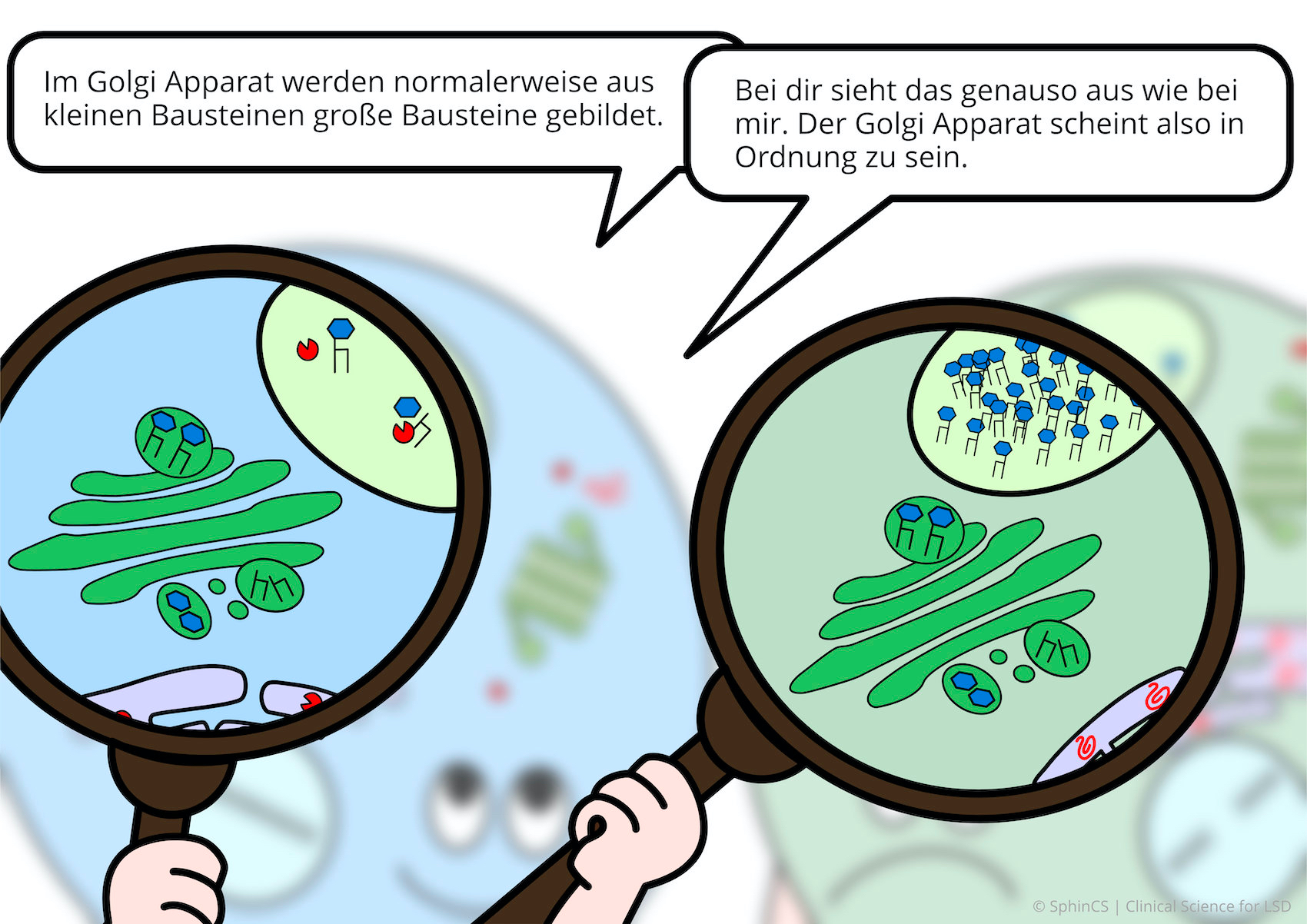
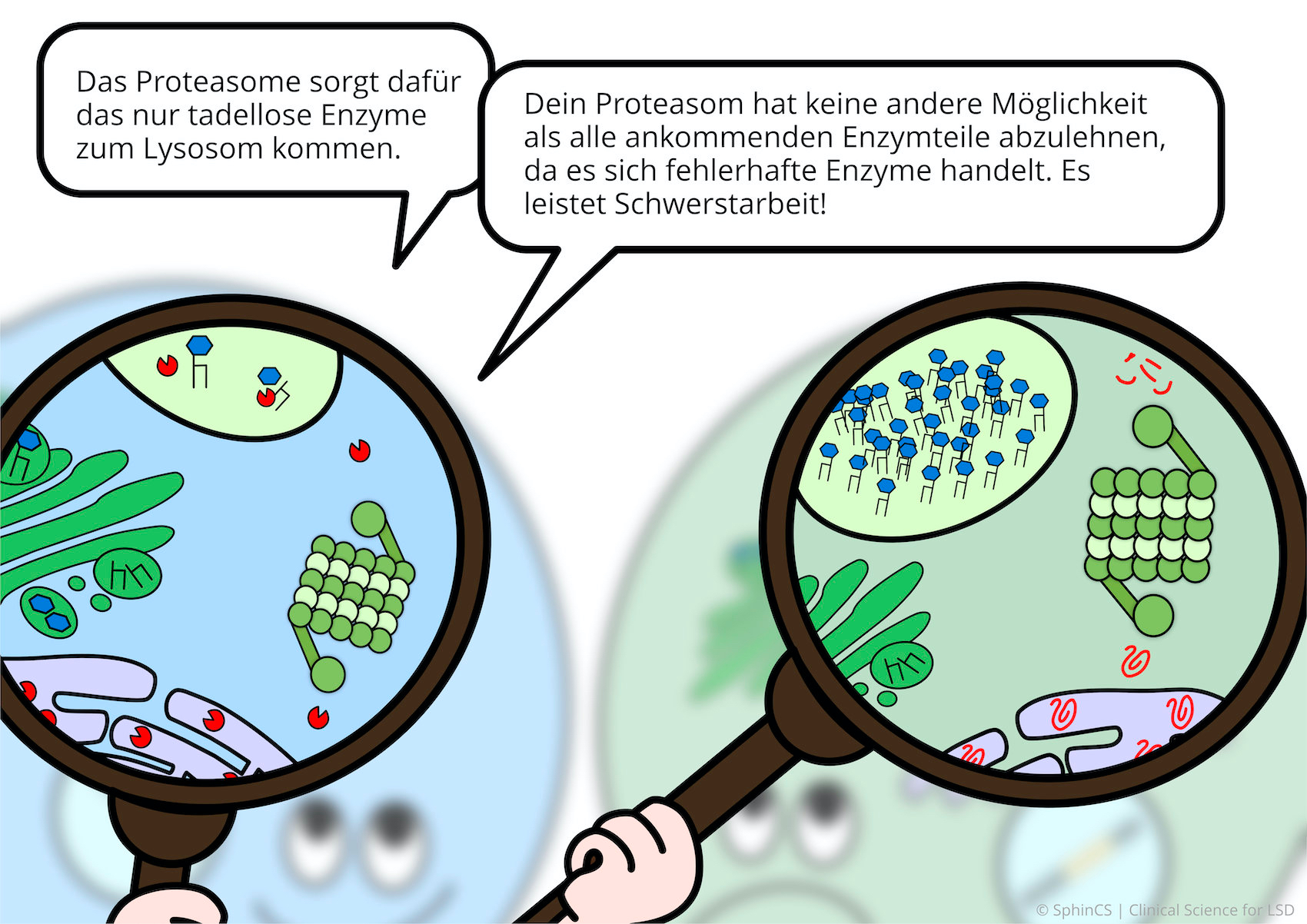
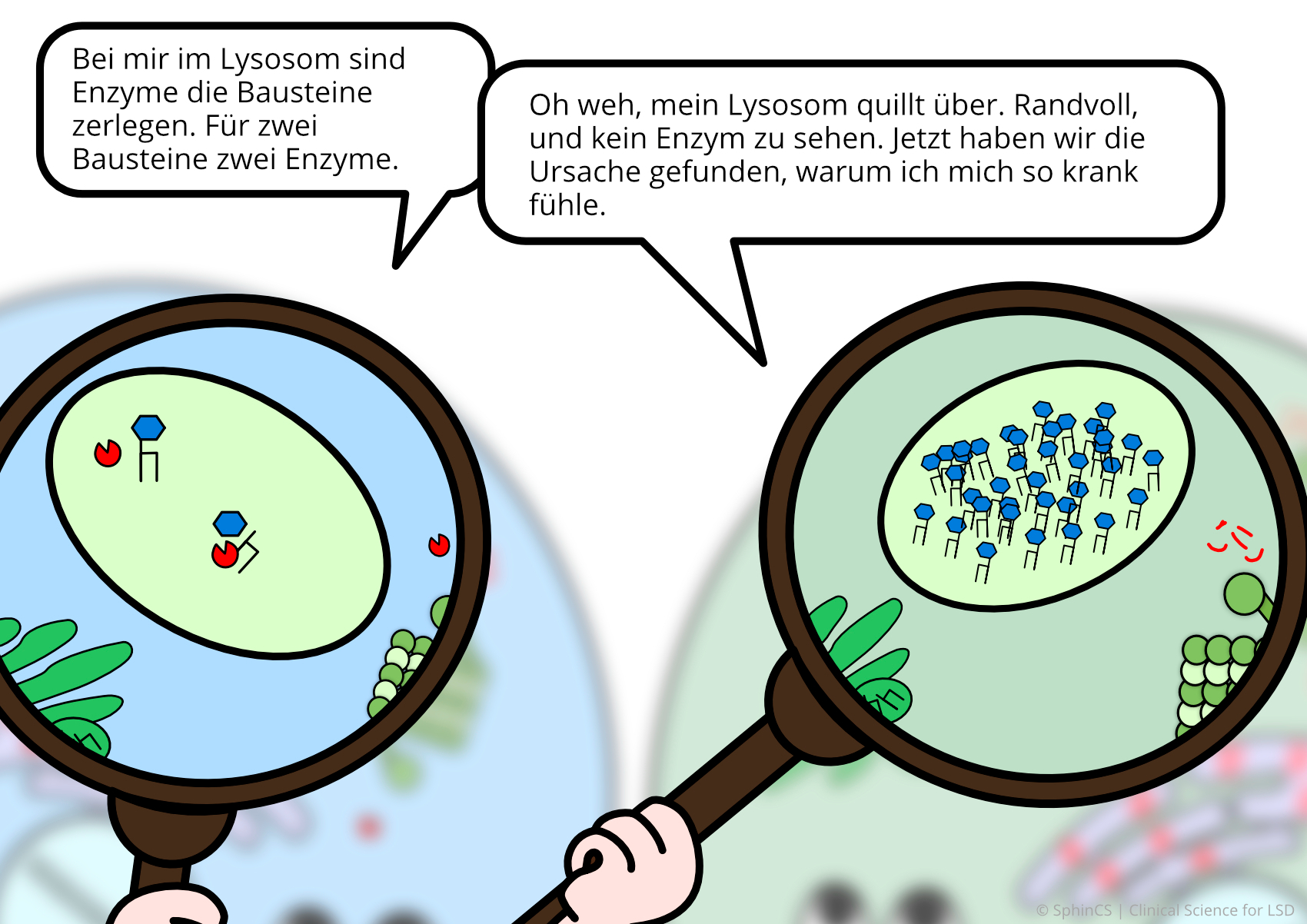
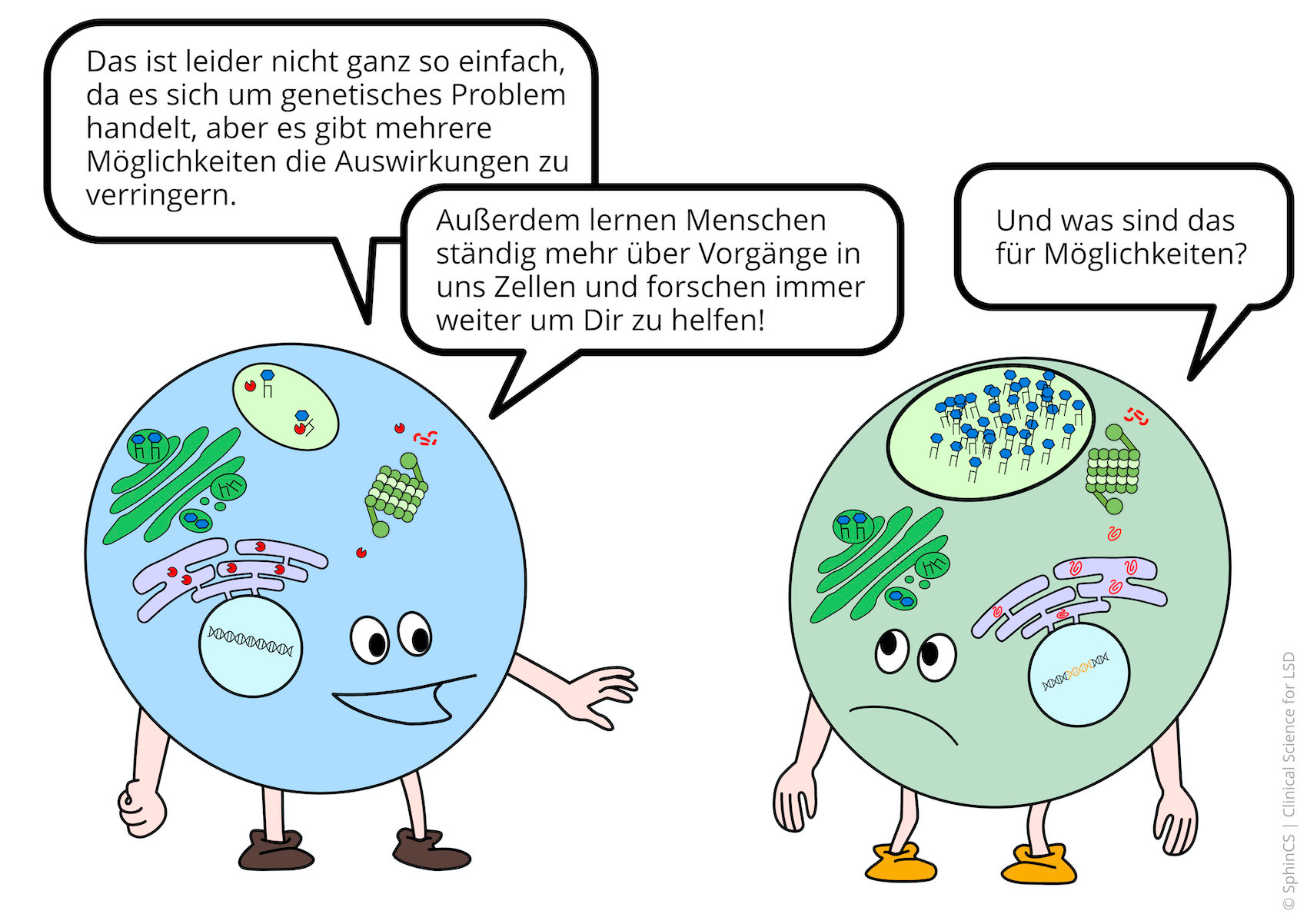
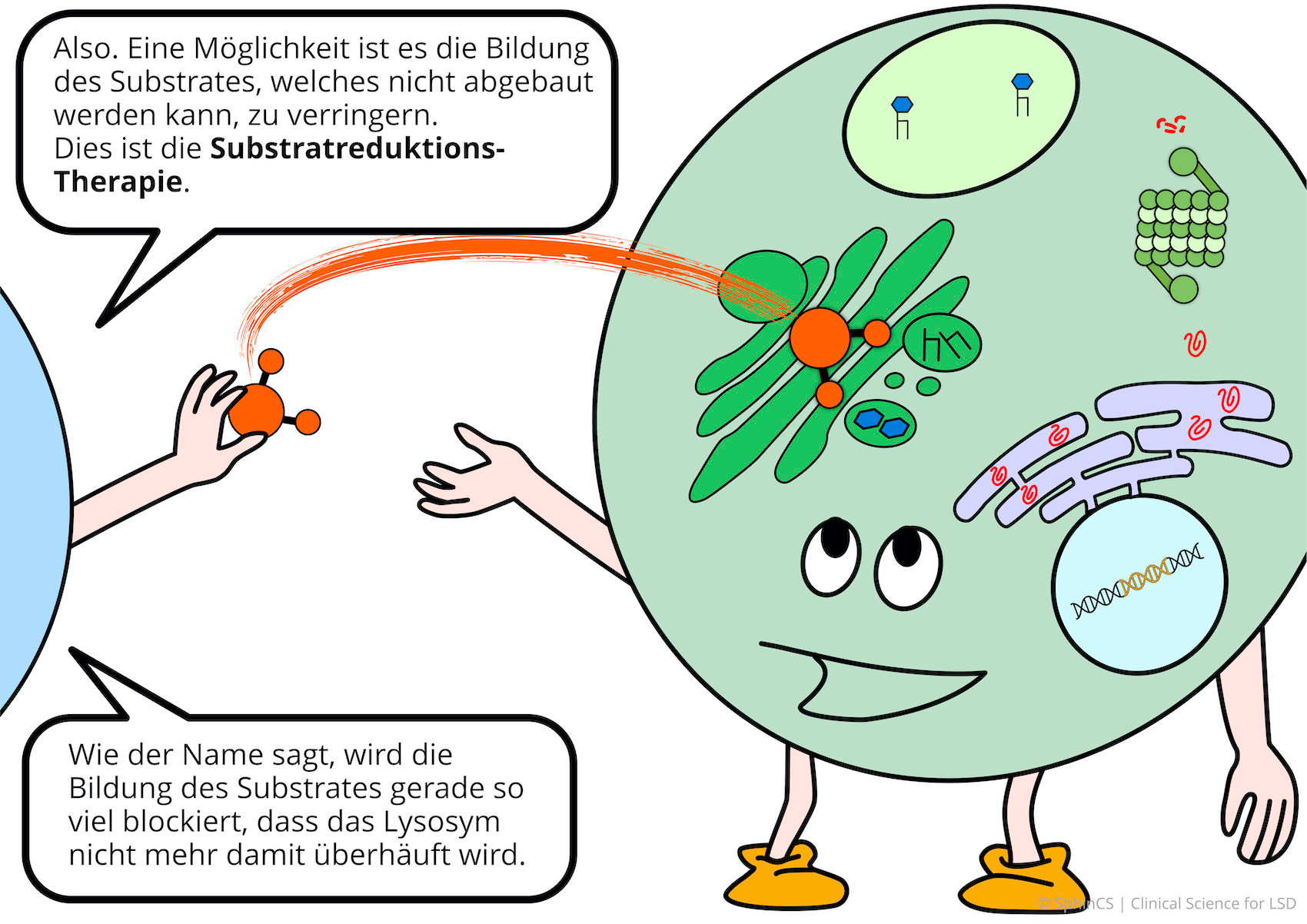
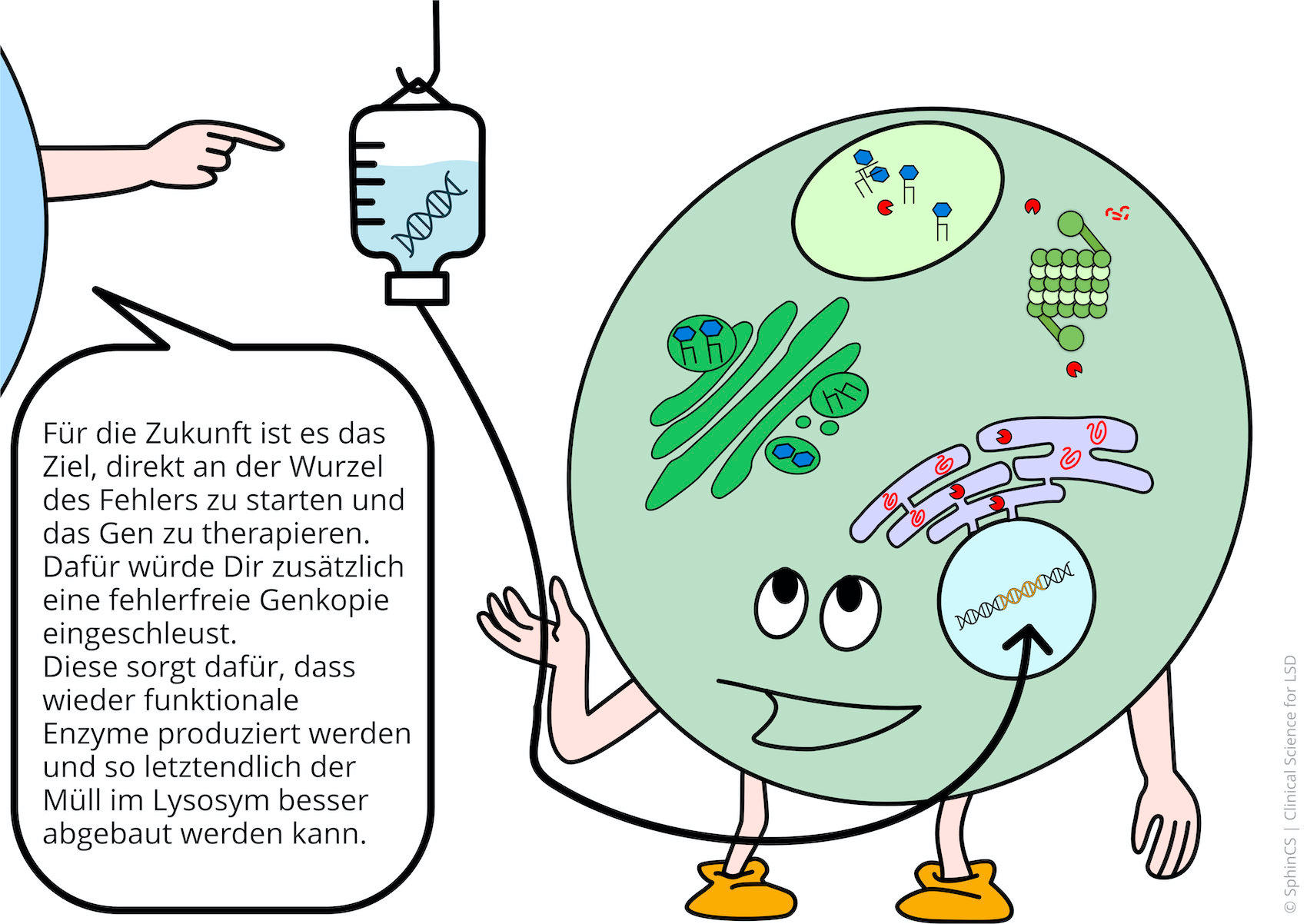
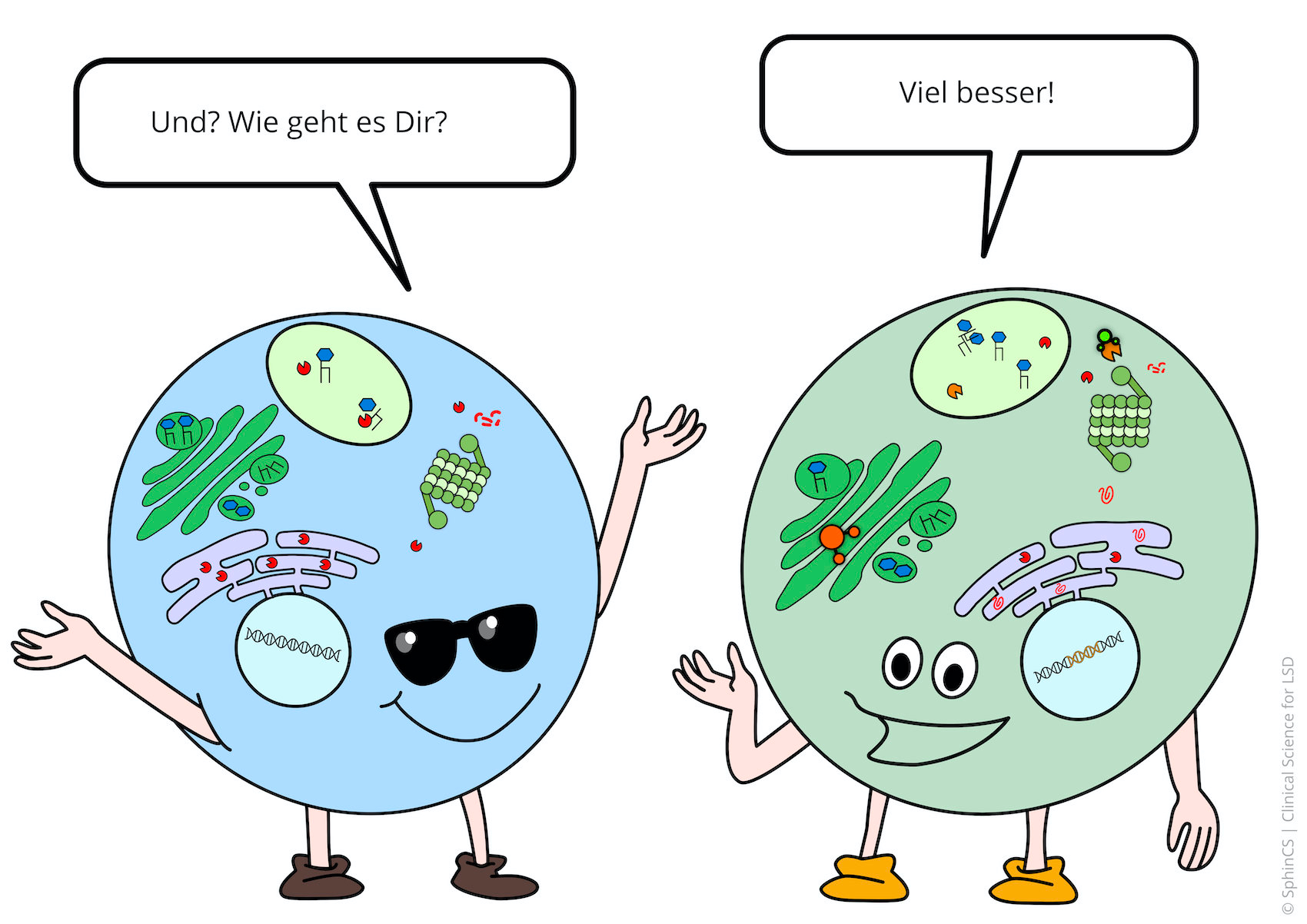
We have illustrated the topic of therapeutic concepts for lysosomal diseases in a comic strip in which we try to explain what is not working in the cell and, more importantly, how it could be fixed.